Understanding Preclinical Toxicology: A Comprehensive Guide for Researchers
Embarking on the journey of drug development is a complex and meticulous process. One of the critical stages in this process is preclinical toxicology, which plays a pivotal role in ensuring the safety and efficacy of potential therapeutic agents. In this article, we delve into the intricacies of preclinical toxicology, providing you with a detailed and multi-dimensional overview.
What is Preclinical Toxicology?
Preclinical toxicology is the branch of toxicology that focuses on the evaluation of the potential adverse effects of a drug or chemical compound before it is tested in humans. This phase is crucial as it helps identify any potential toxicity issues that may arise during clinical trials, thereby saving both time and resources.
![]()
The Importance of Preclinical Toxicology
Preclinical toxicology is essential for several reasons:
-
Identifying potential adverse effects early in the drug development process can prevent costly and time-consuming clinical trials.
-
Ensuring the safety of the drug for human use.
-
Guiding the design of clinical trials to minimize risks.
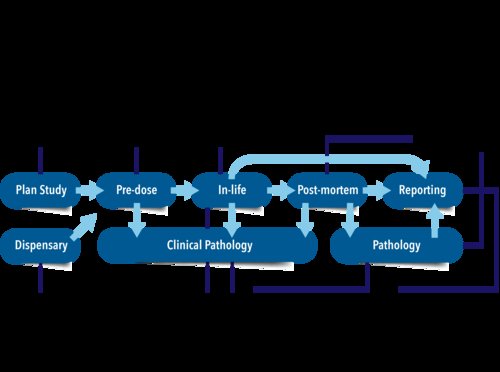
Types of Preclinical Toxicology Studies
Preclinical toxicology studies can be categorized into various types, each serving a specific purpose:
-
Acute Toxicity Studies: These studies evaluate the immediate effects of a drug or compound on living organisms. They help determine the dose that causes toxicity (LD50) and the potential for acute poisoning.
-
Subchronic Toxicity Studies: These studies assess the effects of a drug or compound over a period of time, typically 28 to 90 days. They help identify potential chronic effects that may not be evident in acute studies.
-
Chronic Toxicity Studies: These studies evaluate the long-term effects of a drug or compound, usually over a period of 6 to 12 months. They are crucial for identifying potential carcinogenic, mutagenic, and teratogenic effects.
-
Genotoxicity Studies: These studies assess the potential of a drug or compound to cause genetic damage, which can lead to cancer and other diseases.
-
Immunotoxicity Studies: These studies evaluate the potential effects of a drug or compound on the immune system.
-
Reproductive Toxicity Studies: These studies assess the potential effects of a drug or compound on fertility, pregnancy, and development of offspring.
Methods Used in Preclinical Toxicology
Preclinical toxicology studies employ various methods to evaluate the potential toxicity of a drug or compound. Some of the commonly used methods include:
-
Animal Testing: This is the most common method used in preclinical toxicology. It involves administering the drug or compound to animals and observing any adverse effects.
-
In Vitro Testing: This involves testing the drug or compound on cells or tissues in a laboratory setting. It is less expensive and time-consuming than animal testing but may not always accurately predict the effects in living organisms.
-
Computational Toxicology: This involves using computer models to predict the potential toxicity of a drug or compound. While it is a promising field, it is still under development and not yet widely used.
Regulatory Considerations
Preclinical toxicology studies must comply with regulatory guidelines set by various organizations, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. These guidelines ensure that the studies are conducted in a standardized and ethical manner.
Challenges and Future Directions
Despite the advancements in preclinical toxicology, there are still challenges that need to be addressed. Some of these challenges include:
-
Animal testing remains controversial, and alternative methods are being sought.
-
Interpretation of data from preclinical studies can be complex and may not always accurately predict the effects in humans.
-
The cost and time required for preclinical toxicology studies can be significant.
Future directions in preclinical toxicology












